Accelerate Life Science Validation: From Weeks to Minutes With MasterControl's Innovative Methodology
Get lifesaving products to patients faster by eliminating validation documentation bottlenecks. Our patented risk-based validation tools deliver both speed and compliance for life science organizations like yours.
Tailor your validation to your business needs and to how your business uses MasterControl software.
Learn More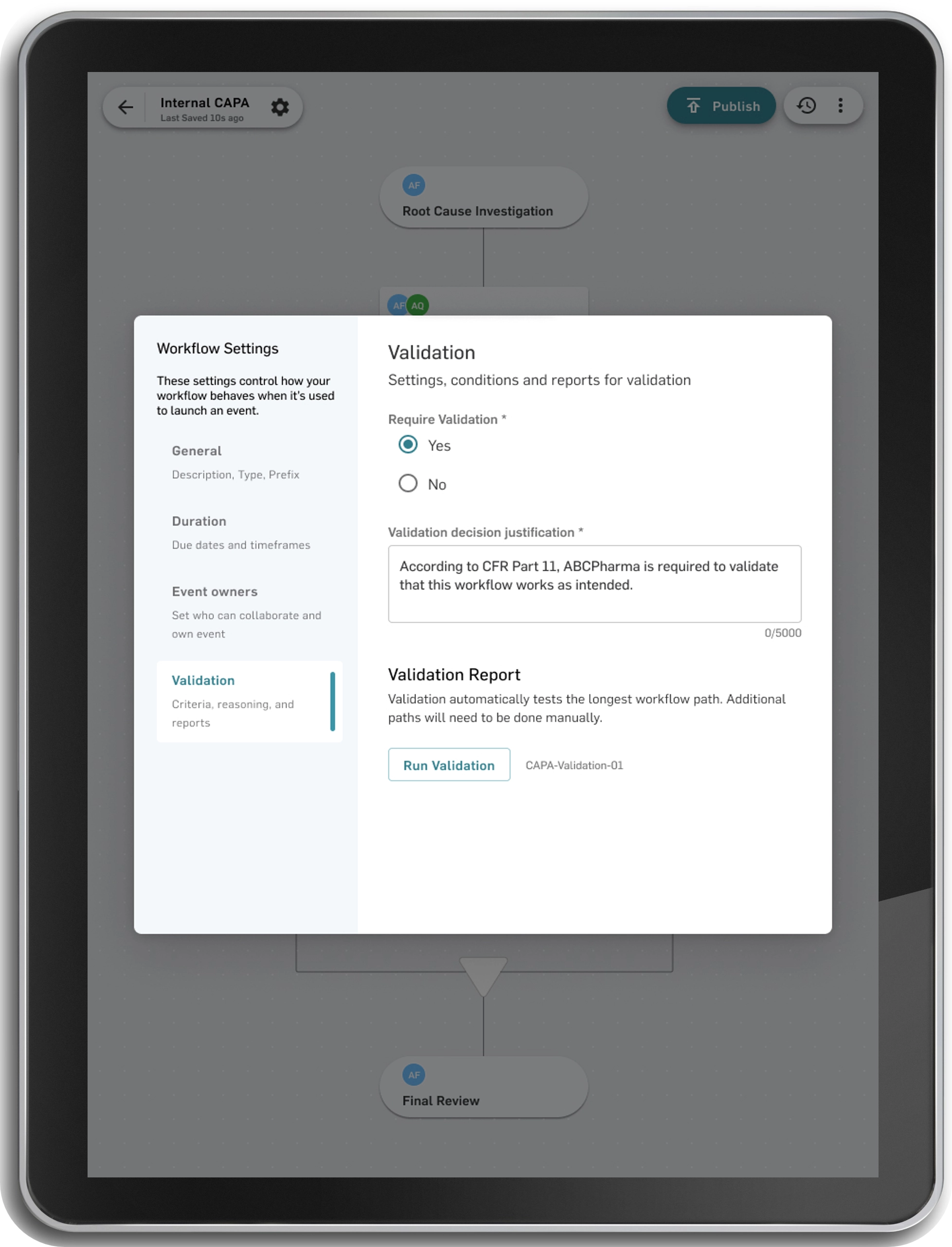
From Emerging Biotechs to Global Pharmaceutical Leaders



{{ sideTopicContent.selectorText }}
{{ sideTopicContent.contentTitle }}
The Results Speak for Themselves:
-
OVER
90 %of MasterControl customers using VxT do no additional formal testing.
-
UNDER
45The average time for upgrade validation is under 45 minutes with MasterControl’s risk-based approach.
-
OVER
330hours saved on average on project preparation means your team can focus on advancing science, not documentation.
Wellington Foods Case Study:
Master Validation with VxT
Discover how a leading manufacturer of quality liquid and powder based dietary supplements leverages VxT to focus validation precisely where it matters most.
Watch the Success StoryMaster Change Control
Built-in validation transforms release and change control processes while maintaining impeccable GxP compliance.
Master Upgrades
Only review new features and functionality that affect your critical business processes with each software upgrade.
Ready for Any Inspection
Generate comprehensive documentation for FDA, EMA, and other regulatory inspections, supplier audits, or customer quality agreements.
Powerful Functionality, No Hidden Costs
Industry-leading validation tools embedded in your MasterControl platform at no additional cost.
Validation Services That Go Further
Specialized Validation Services for Life Science's Unique Challenges
Our validation experts provide specialized services for integration utilities, custom form validation, data verification qualification, and personalized consultation tailored to your specific regulatory requirements.

Additional Resources
Use our collection of resources to learn more about validation and the value of taking a risk-based approach to validation to save time and maintain compliance.
See Validation ResourcesDiscover a faster way to validate.
Learn how our cutting-edge tools and start-to-finish validation solutions and services can help you reduce the time, effort and costs involved in validating your systems.

