


Software That Eliminates Trade-Offs
Bringing breakthrough products to market in highly regulated industries can feel like an endless series of trade-offs. MasterControl's software simplifies GxP workflows so you never have to sacrifice quality for cost or innovation for regulation.
Quality
Cost
Speed
Safety
Control
Flexibility
Innovation
Regulation
Quality
Management
System
Complete and connected meets fast and flexible. From quality event management to document control and integrated training - MasterControl Quality Excellence transforms your quality data and processes into a competitive advantage.
- Quality Events
- Document Control
- Training and Exams
- Audit Management
- Risk Management
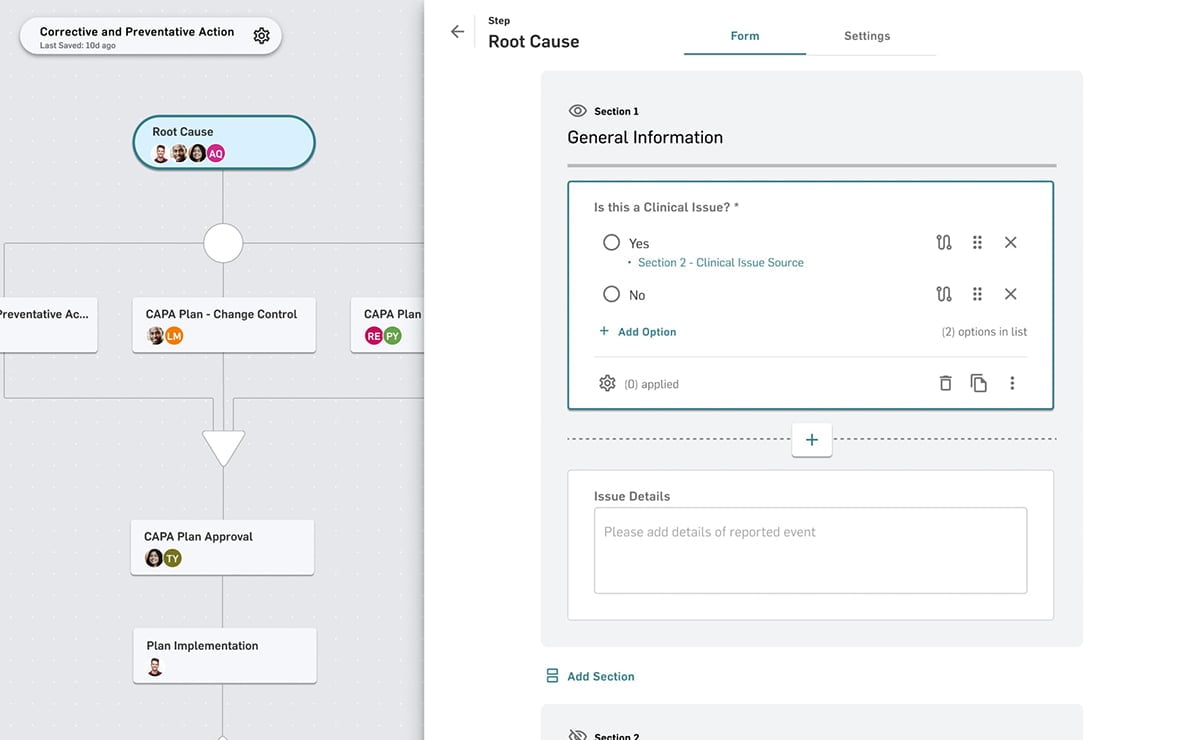
Manufacturing
Execution
System
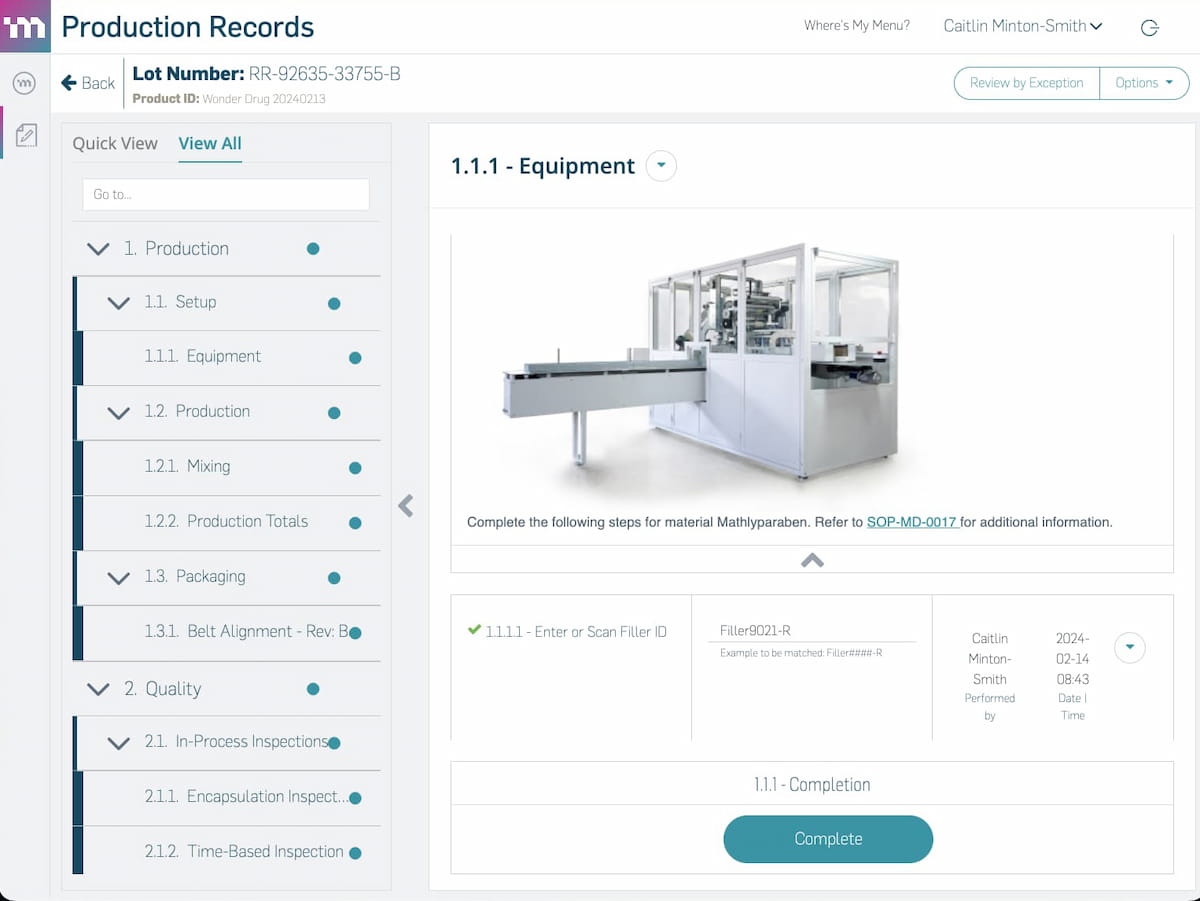
Modernizing your manufacturing operations starts with modern software. From work orders to work instructions and production records (EBR or eDHR) to logbooks, MasterControl Manufacturing Excellence is the simplest way to digitalise manufacturing.
- Manufacturing Execution
- Work Instructions
- EBR or eDHR
- Logbooks
- Integrations
Results You Can Measure
Our customers experience incredible value when moving their quality and manufacturing processes to the MasterControl platform. Read their stories and see the transformative value created by these major players in the pharma, med device, and nutraceutical industries.
View All Case Studies