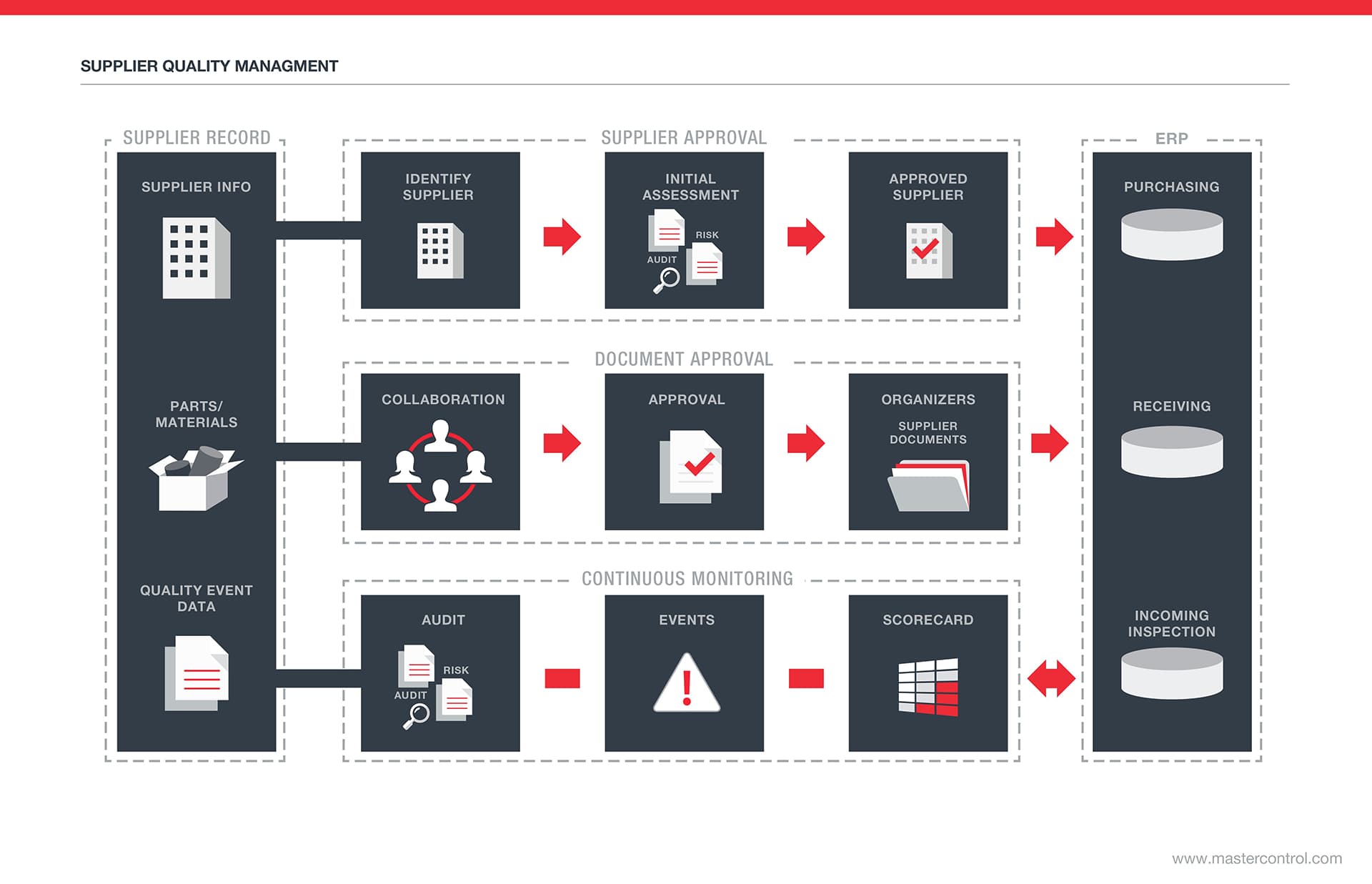
Supplier Quality Management Software
As supply chains grow more sophisticated and global, they also become increasingly vulnerable to a wider range of risks, including disruptive events like natural disasters. Despite the increased risks, and the profound effect they have on business performance and shareholder value, a recent survey of more than 500 manufacturing executives found that only two thirds of companies have a supplier quality management system in place, and only half of the surveyed executives feel their existing system is effective. Companies that are without a system, or those that are relying on outdated tools, are at a major competitive disadvantage. The use of electronic supplier quality management software can help global companies create stronger, more resilient supply chains.