GxP Lifeline
How Electronic Batch Records and Device History Records Close the Digital Manufacturing Gap

Game-changing tools around big data and advanced analytics are enabling pharmaceutical manufacturers to process unprecedented amounts of data for improved decision making. Yet even as life sciences manufacturers invest heavily in enterprise and plant automation, their efforts are too often impeded by a digital manufacturing gap, where critical production processes remain paper-based and disconnected.
In a recent webinar poll of pharmaceutical professionals(1), 79% of attendees said they are using paper production records – aka batch records or device history records – while 21% reported having some level of electronic production records. To help explain the latter statistic, it’s helpful to understand the ongoing role of paper in life science manufacturing:
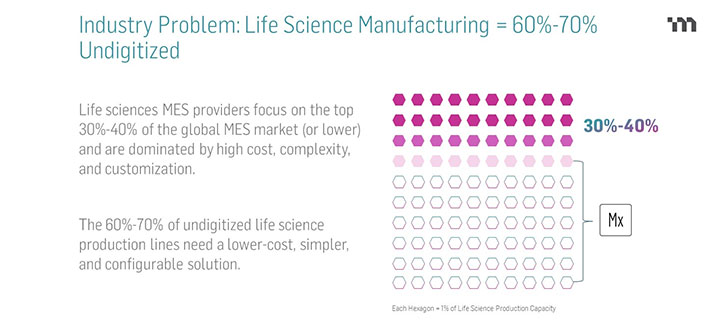
We need to remedy the fact that the vast majority of life science manufacturers are still on paper. To support advanced data technologies and to compete in a modern market, manufacturers must close the digital gap on the shop floor.
Digitizing the paper-based production record process can close this gap by creating productive connections between people, processes, and systems throughout the manufacturing process and across the entire enterprise for a holistic view of production data.
Digitize for Connected Operations
Many manufacturers already leverage a number of software solutions. Common enterprise applications in a manufacturing IT ecosystem include enterprise resource planning (ERP), learning management system (LMS), quality management system (QMS), supervisory control and data acquisition (SCADA), and more. It’s fairly common to hear the term “islands of automation” because a paper-based production record doesn’t pull together the data from these systems.
All of these systems are generating, consuming, and outputting data, but where’s your data for your production record? It’s locked away, in paper, that’s probably in bankers’ boxes stored in some secure location.
What’s missing is the production record solution, which creates a digital thread that pulls everything together.
The digital thread connects all of these systems and this information, which is key.. The digital thread gives you context in the frame of the production record. All of the other systems are critical, but they don’t do the production record, which is still on paper far too often.
By integrating these disparate, but critical, software applications with a fully digital production record solution like MasterControl Manufacturing Excellence, manufacturers can collect data while maintaining data integrity; share data between those systems and functional areas completely, seamlessly, and immediately; and contextualize the data to make better business decisions.
Visibility With Digitized Connectivity
Pharmaceutical manufacturers know their processes, document their processes, follow their processes, and iterate on those processes better than most industries. They have to because they have a higher degree of scrutiny. But all of the data resulting from execution of those processes is put on paper when you’re using a paper-based production record.
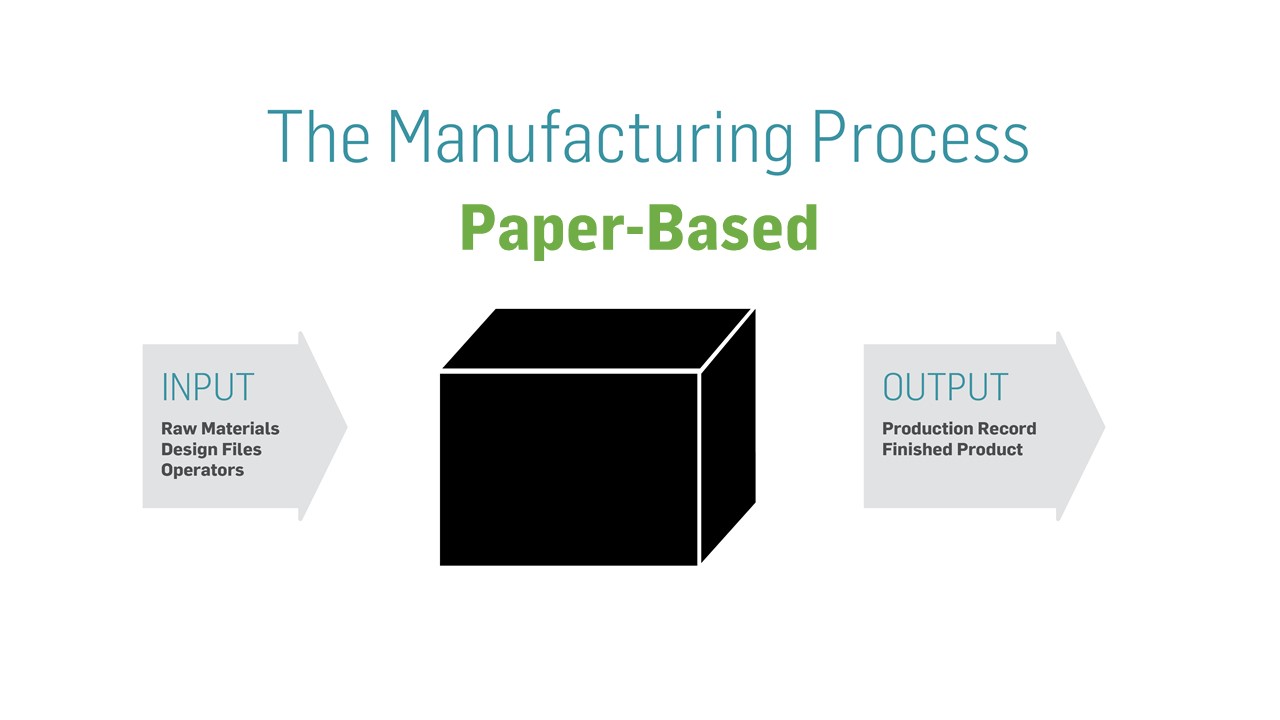
If you look at the manufacturing process as having a beginning, middle, and end, what manufacturers tend to know best are the beginning, the end, and the as-designed middle. The beginning would consist of the inputs – raw materials, work instructions, and operators, for example. The end would consist of the outputs – yield, scrap, paper production record, and finished product, for example. The as-designed middle is the master template and work instructions that document the overall manufacturing process.
However, the as-executed production record is stashed away in bankers’ boxes after the current production record completes. Knowing what happened with previous production runs or with production runs at different sites and/or shifts is like looking at a black box. This valuable data is trapped on the paper and inaccessible.
Digitizing the production record provides visibility into the manufacturing process and as-executed data that was previously locked up in paper, including data relating to lines, batches, or lots. Comparative analyses can now be done to support continuous improvement efforts. Comparisons can now compare today’s execution to those yesterday, three months ago, last year, and so on. Similarly, comparisons can be performed between sites, shifts, teams, and so on, unlocking the data needed to improve.
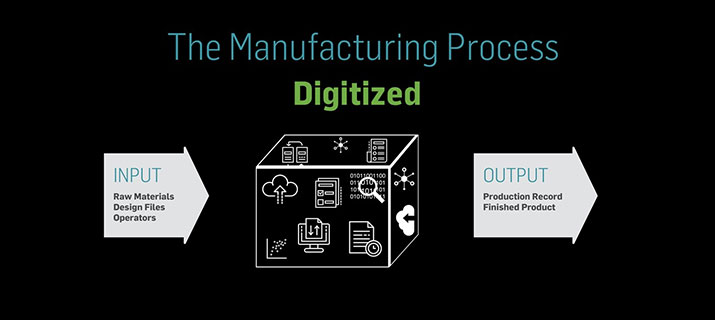
The end-to-end visibility that comes with fully connected production records allows access to new, previously missing datasets in digitized records, including which operator performs a step the fastest with fewest errors or which shift outperforms others.
Outcomes of Digitally Connected Operations
Closing the digital manufacturing gap by digitizing paper-based production record processes dramatically reduces the human errors inherent with paper and goes beyond that to provide many more business results, including:
- Streamlining production processes, reducing delays and downtime, and enforcing quality controls without slowing production. Using MasterControl Electronic Batch Records (EBR), Gritstone Oncology and other customers scaled their organizations with 30% fewer people. Wellington Foods, like many other customers, saw a 90% decrease in data input errors using the manufacturing solution.
- Reducing the number of deviations and quality events to manage and being more efficient at managing those that do occur. MasterControl customers for decades have used MasterControl’s quality management solutions to more efficiently process quality events, such as deviations. Even better is reducing the total number of deviations that need to be processed. For Wellington, most deviations today are planned deviations, rather than unplanned, due to inventory issues/supply chain disruption and can be managed in the master batch record. MasterControl Manufacturing Excellence all but alleviated the unplanned deviations that were formerly generated by human error triggered by the paper problems.
- Achieving faster time to market by improving collaboration, reducing quality review time, and accelerating product release. Using MasterControl’s EBR solution, QuVa Pharma has been able to better align and reduce friction between QA and production, resulting in faster review/release cycles. Wellington reduced its production review cycles from several weeks to several hours.
Conclusion: Digitize to Build Quality Into the Manufacturing System
Quality should be built into the manufacturing system rather than outside of it. Manufacturing professionals may bristle at this statement, as they’re just as interested as quality professionals in building quality into manufacturing and building a quality product. However, if they’re still using paper, there is more they could and should do to deliver higher-quality products.
them to be right. By improving data integrity, increasing visibility, and optimizing production processes, fully connected, digital production records close the digital manufacturing gap that persists on the shop floor – ultimately giving manufacturers access to better data to drive better, smarter business decisions.
Resource:
- “Adopting Technology in the Quest for Digital Transformation,” European Pharmaceutical Review Webinar, Brian Curran and Katie Farley, October 2021.

