
GxP Lifeline
OCM and eQMS: Transformation or Conformance?

In this second of a three-part series of articles (read the first installment here), we will continue to discuss electronic quality management systems (eQMS) and how to establish a strong foundation for transformational change. Let’s pick-up our discussion from the first article.
The first article in this series ended with four foundational activities to fuel your organizational change management (OCM) strategy and plan. We’ll explore each of the four steps and why they are imperative to the success of your eQMS and overall digital quality control efforts.
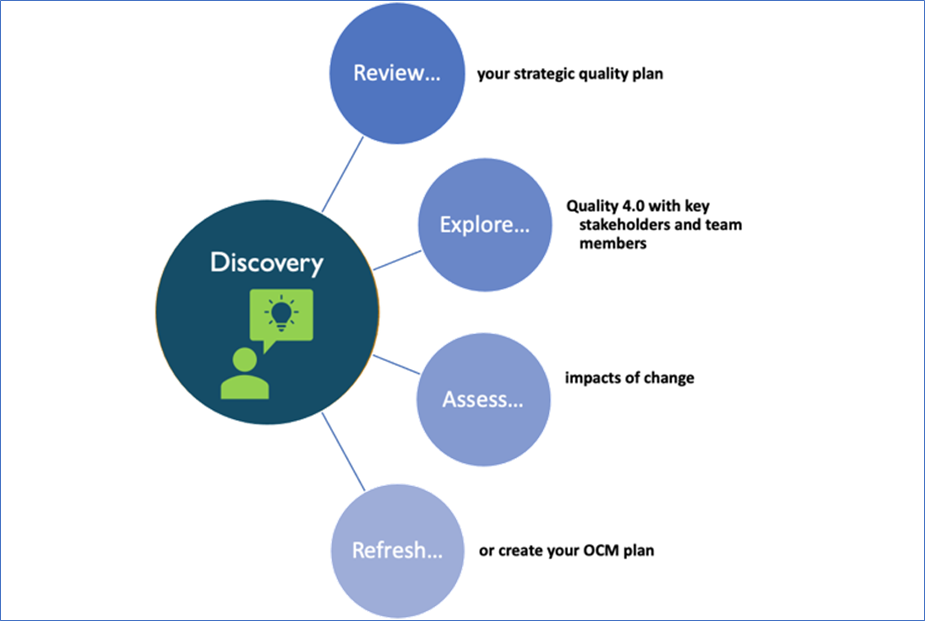
Step #1: Review Your Strategic Quality Plan
This is where the connection between the strategic goals of an organization and how the eQMS supports those goals can be made. Plans that address quality as well digital strategy, adoption of advanced technology, and technical standards, contain insights for the OCM plan. Major technology investments are connected to organizational goals and making that connection for people can accelerate adoption. When people understand these connections, they can embrace the strategic implications for an eQMS and are more likely to adopt the changes that may be seen as disruptive to the norm.
Strategic plans help build the foundation for the OCM plan. And while strategic plans don’t address technology or eQMS digitization explicitly, quality leadership plays a key role in connecting the eQMS to the “greater goals” of the organization. Failing this, projects may have a rough start, appearing fragmented from the corporate goals and viewed as a conventional technology project instead of one that is highly innovative.
John Flaherty, a Peter Drucker management expert and author (2002), provides a valuable perspective on organizational strategy as being traditional, transitional, or transformational. Flaherty explains that “the traditional business question is: what is our business? The transitional business question is: what will our business be if current trends continue? The transformational business question is: what should our business be?” Reviewing your strategic quality plan will help you to understand where your organization falls. Quality leaders can be the strongest advocates for change when it comes to understanding their organization in the context of striving to be transformational or somewhere in between.
Step #2: Explore Quality 4.0 With Key Stakeholders and Team Members
Quality 4.0 contains valuable insights for how to plan and design your eQMS project. It is intentionally disruptive as it includes innovative strategies that leverage advanced technologies. Adopting a Quality 4.0 mindset is timely as the life science industry must resolve ongoing challenges in quality that stem from lack of data-driven processes to enable decision-making, cultures that do not share the same standards for driving a quality mindset, and a lack of transparency into cross-discipline processes. Exploring these concepts is vital to your eQMS strategy and roadmap.
Step #3: Assess the Impacts of Organizational Change on Your People
The enormity of change for your people can be daunting if you are disrupting the traditional model. Unfortunately, not everyone will respond well to radical change. An important OCM technique is to execute a Change Impact Analysis (CIA). The focus of the CIA is to understand what people are expected to do in the future state in terms of process and technology change. The CIA is a critical deliverable and cross-discipline effort between OCM, IT, project management, vendor resources, quality leaders, and end users. Pilots or role-based scenario testing can be highly effective to clarify the impact of eQMS digitalization on your people.
Step #4: Refresh or Create Your OCM Plan
The prior steps provide inputs for the OCM plan and are integrated with specific OCM activities. A comprehensive plan includes outcomes and actions including:
-
Stakeholder Assessment
-
Change Impact Analysis
-
Leadership Identification and Engagement
-
Communications Plan
-
Change Interventions
Who is being impacted, where are they, what are their roles, and how are they involved in the new eQMS?
Dependent on the actual eQMS, a CIA is performed as early as possible and refreshed as the project unfolds.
Confirms the leadership coalition and activities to align quality and business leaders through face-to-face briefings, monthly publications, demonstrations, etc.
Only one of the deliverables the OCM team brings to the table. Change is driven through consistent messaging formulated at the start of the project about what is happening and what people should expect now, in the mid-term, and in the future; audiences, frequency, and channels (distribution methods) are defined.
These may include other forms of communications (non-written) with specific functions and the formation of a Change Champion network that should be led by a key quality leader.
We covered a lot of ground in this part. In the final piece, we will summarize and end with OCM best practices and examples (from real life projects) for how you can lead change in this critical transformation for life sciences! Part three will explore these major steps in the OCM lifecycle.
References:
- Flaherty, J.E. 2002. Peter Drucker: Shaping the Managerial Mind--How the World's Foremost Management Thinker Crafted the Essentials of Business Success.
