Rethink What You Know About Shop Floor Technology
An MES system isn't the only path to digitizing and automating the shop floor. In fact, for most production environments, a traditional MES is too expensive, complex, and rigid to implement and is only installed on high-volume and high-margin lines. MasterControl’s Manufacturing Excellence solution is disrupting the market with a modern, cloud-based alternative that is fast to deploy, simple to configure, easy to use, and cost-effective to roll out across all your lines and sites.
Rapid Implementation and Adoption
Don’t let implementation time and associated costs stop you from going fully paperless. Unlike a traditional MES that is expensive and time-consuming to implement, MasterControl’s cloud-based manufacturing solution is cost-effective and fast to deploy.
-
6 weeks to implement
-
2 weeks to train users
-
4–8 months to achieve ROI
Flexible, Configurable System
Forget months of building master records and endless consultant hours to manage and maintain your system. MasterControl’s highly configurable solution lets you quickly digitize your existing processes and easily build, manage, and execute production records.
-
Build master records in hours
-
Easily manage parameters
-
Quickly scale lines and sites
Powerful Production Data
Don't let data trapped in paper or legacy systems slow your operations. MasterControl's MES system integrates production data and processes with your critical business systems. Analytics and in-process dashboards provide real-time operational visibility and optimization.
-
Right-first-time data
-
End-to-end process visibility
-
Robust analytics and data export
Resolve Common Manufacturing Challenges
MasterControl's MES system Manufacturing Excellence is designed to remove the complexity and uncertainty in manufacturing execution. It lets you connect, streamline, and manage all your manufacturing information using a single solution.
{{card.title}}
{{card.desc}}
Manufacturing Excellence customers have dramatically improved efficiencies. So can you.
Calculate Your ROIWhat You Get With MasterControl's Manufacturing Excellence
MasterControl’s digital tools enable smarter, faster manufacturing. Make immediate performance gains at every step.
Production Planning
Plan and organize your manufacturing projects with the right tools to ensure your teams are effective and improve production accuracy.
Easily manage product variations and recipes, substitutions, and change control.
Automate materials and work order data between production records and an ERP.
Quickly author, launch, and manage configurable production record templates.
Link SOPs and work instructions to ensure the correct versions are always used.
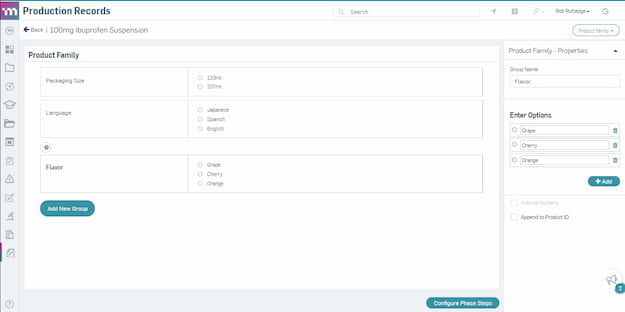
Product family configuration on a master template.
Manufacturing Execution
Accelerate product release by optimizing manufacturing production processes and reducing delays and downtime.
Ensure up-to-date digital work instructions, with real-time tracking of stages, steps, and performance.
Achieve right-first-time production, every time, with automatic data integrity checks.
Enforce limits, controls, and thresholds using integrated, data-driven prompts.
Gain real-time work-in-progress visibility and traceability with in-process dashboards.
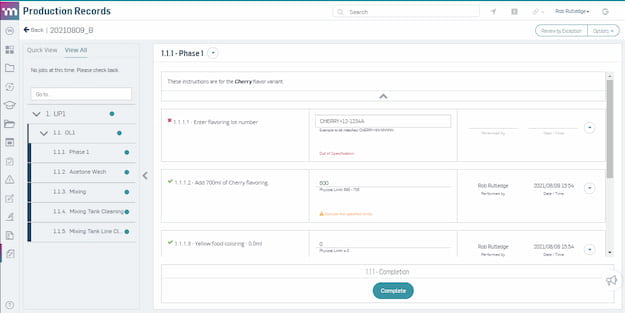
Quality Control and Assurance
Ensure quality at every step of production. Automatically assess risk and launch quality events directly from the production line without slowing production.
Enforce in-line quality assurance and operator training compliance.
Automate and easily manage QC sampling/testing and QEM via LIMS.
Automate equipment calibration and maintenance processes, including scheduling.
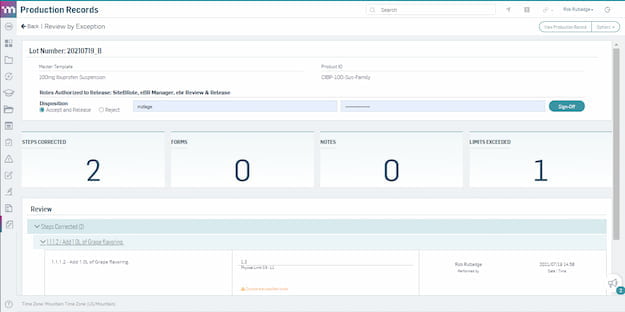
Accelerate production record review and approval with review-by-exception.
Data Management and Analytics
Close information gaps caused by disconnected systems and paper production records. Unlock real-time insights and unleash powerful intelligence.
Proactively track production data and resolve data integrity issues before they spread.
Access data on shop floor performance using operational or role-based dashboards.
Connect systems, processes, and people for a complete view of data and actionable insights.
Apply advanced analytics using machine learning/AI to make data-driven improvements.
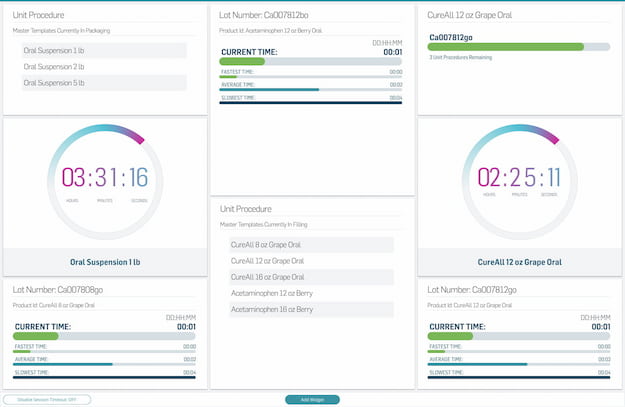
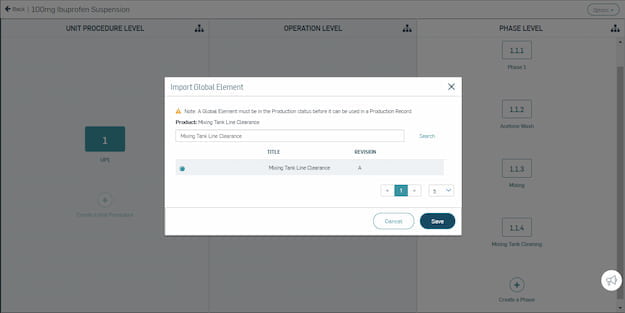
Master Record Management
Significantly reduce time spent building master record templates, and automate version change control. Master your digital information management.
Easily create configurable, flexible templates for production records in a digital system.
Leverage global elements, templates, and a product family tool to manage recipes and variants.
Automatically track and store all relevant records electronically with a compliant audit trail.
Flexible Pricing for a Scalable MES Solution
MasterControl Manufacturing Excellence delivers the agility and scalability needed to support change and growth for companies of all sizes. With flexible pricing plans, MasterControl modern MES solution is far more cost-effective than traditional manufacturing software. That means you gain more while spending less.
Any Business Size
MasterControl meets the needs of small startups, global enterprises, and every size company in between.
Grows with You
The MES solution grows as your organization grows. You can seamlessly upgrade and meet increased demand.
Cloud-based Management Software Solution
Our cloud-based solution with flexible plans ensures you have all the tools you need to get the job done right.
Learn How to Achieve Smarter, Faster Quality Manufacturing
See how a flexible, fully connected manufacturing solution allows manufacturers to realize the benefits of real digital transformation.